Researchers at the University of Regensburg and Graz University of Technology have shown that hydrogen atoms on the sides of molecules on the surface can be imaged directly. The study, recently published in the journal Proceedings of the National Academy of Sciences, describes that by looking next to molecules, the location and presence of previously hidden hydrogen atoms can be revealed.
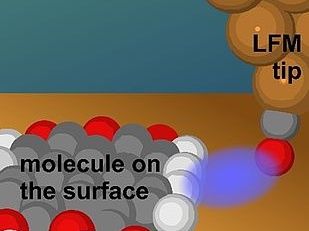
Artist's impression of an LFM tip approaching the side of the molecule where it is sensitive to the terminal H atoms.
© AJ Weymouth
Hydrogen atoms, located on the outside of molecules, affect many properties of those molecules, including how they interact with other molecules. Hydrogen bonds are one of the most common forms of molecular interactions, in which a positively charged hydrogen atom on one side of a molecule is attracted to a negatively charged atom on an adjacent molecule. Hydrogen bonds are of great importance in the field of surface synthesis, where molecules are first adsorbed on the surface and then interact with each other. But despite their importance, direct observations of these small but important atoms have been nearly impossible until now.
To image the sides of the molecules, the researchers used a special technique derived from atomic force microscopy (AFM). In AFM measurements, a sharp tip is brought close to a surface and the forces acting on the tip as it moves across the surface are recorded. Previous AFM experiments focused on the vertical component of the force and were therefore unable to detect hydrogen atoms on the sides of the molecules. To overcome this limitation, the researchers used lateral force microscopy (LFM), which measures horizontal forces acting on the AFM tip. Dr. BD. Alfred J. Weymouth from the work group of Professor Dr. Franz J. Giesebel, head of the Department of Quantum Nanoscience at the University of Uruguay, is one of the leading experts in the field of LFM. Highlighting its unique capabilities, he explained: “Although not yet widely used, LFM offers several advantages over traditional AFM, including exceptional distance sensitivity that allows physical parameters to be extracted from a single image and the ability to measure… Frictional forces, by pushing a single atom through chemical bonds.
By measuring the lateral force applied to the AFM tip at the edges of the particles, Dr. Weymouth and his colleagues made hydrogen atoms directly identifiable. Raw data from experiments can be compared directly with theoretical calculations, leading to a better understanding of atomic interactions. While atom–atom interactions are often modeled using simplified distance-dependent functions, comparison of these models with experimental data has highlighted the limitations of these approximations and highlighted the importance of incorporating additional factors into these theoretical frameworks.
These results are valuable for both AFM and LFM studies because they allow researchers to improve their understanding of fundamental atomic interactions.
The ability to directly observe hydrogen atoms represents a major research breakthrough and provides a powerful tool for elucidating the complex mechanisms and intermediate steps of chemical reactions on surfaces. This achievement holds enormous potential for progress in various fields, including surface catalysis and molecular interactions in the human body. The development of this new technology expands our understanding of the microscopic world and opens new horizons for research and innovation. By directly visualizing the behavior of hydrogen atoms, researchers can gain deeper insights into the fundamental processes that govern molecular interactions, paving the way for transformative advances in various fields.

“Subtly charming coffee scholar. General zombie junkie. Introvert. Alcohol nerd. Travel lover. Twitter specialist. Freelance student.”






More Stories
Data Leakage: Android TV can expose user's emails and files
How did life begin on Earth? Munich researchers find important clues
The “One-Man-Show” Next-Gen Update shows how to please players